Prescribing information and Adverse event reporting can be found at the bottom of the page
Search Header
Allows placement with one hand for all Bayer IUS devices
Designed with healthcare professionals and patients in mind
Healthcare professionals’ and women's concerns about difficult insertion or pain associated with placement are barriers when considering intrauterine contraception. By involving healthcare professionals in the development process, the next evolutionary step in modifying the inserter has been made possible.
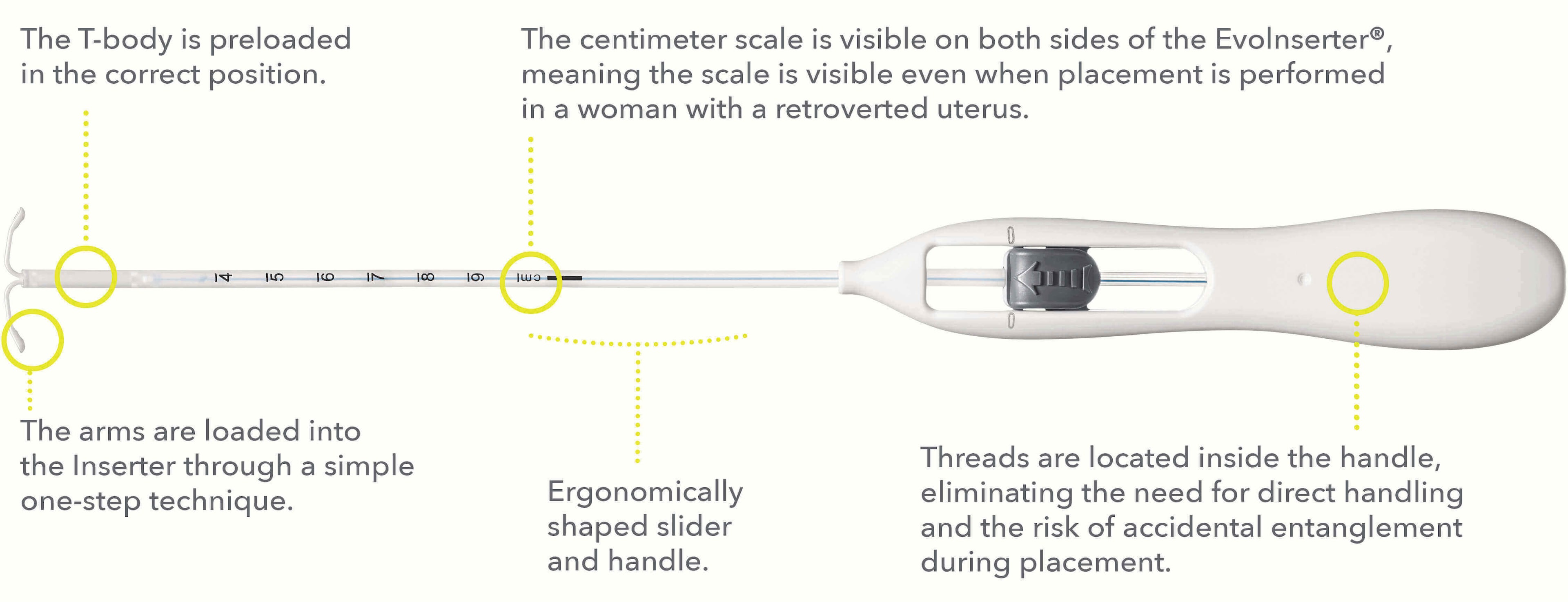
The Bayer EvoInserter® offers a reduced number of preparatory steps and allows placement with one hand, because it is essential to focus on what is most important: taking care of the patient during the placement procedure.
For Bayer EvoInserter™ the insertion technique for placement of all three Intra-uterine systems (IUS’s) is identical

How to load the IUS into the insertion tube
The T-body is already preloaded in the correct position, simply push the slider forward in the furthest position to load the IUS into the insertion tube.

How to deploy the IUS after insertion
Pull the slider to the mark to open the horizontal arms and wait 5-10 seconds for the horizontal arms to open completely.

How to release the IUS
Release the IUS by pulling the slider all the way down.

Demonstration video
Important information: Use these animation videos only as additional information. For complete insertion instructions, please refer to the product information for prescribers.
>42M
placements have been performed with EvoInserter1
Insertion Tube Diameter:
Kyleena® (19.5mg levonorgestrel) & Jaydess®▼ (13.5mg levonorgestrel) = 3.8mm
Mirena® (52mg levonorgestrel) = 4.4mm
- Beckert V et al. Eur J Contracept Reprod Health Care 2020;25:182–189. Return to content

Explore Bayer IUS products that use the EvoInserter
Latest content
PP-PF-WHC-GB-1031 May 2024
Reporting adverse events and quality complaints
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Bayer plc.
If you want to report an adverse event or quality complaint, reports can be directed to: Tel: 01182063500 or email: pvuk@bayer.com
Further information is available on the “contact” tab at www.bayer.co.uk.






